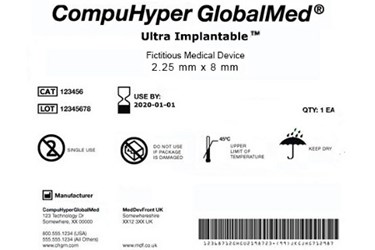
Include a unique device identifier udi issued under an fda accredited issuing agency s udi system on device labels device packages and in some instances directly on the device.
Fda unique device identification udi rule.
Food and drug administration fda created unique device identification often abbreviated udi a rule that requires medical device manufacturers to update their products with a unique device identifier that includes both device and production identifiers such as expiration date and lot or serial number.
The unique device identification system final rule udi rule requires device labelers typically the manufacturer to.
Include a unique device identifier udi on device labels and packages.
Unique device identification system.
On september 24 2013 fda published a final rule establishing a unique device identification system the udi rule.
Unique device identification udi the u s.
The system established by this rule would require the label of medical devices and device packages to include a unique device identifier udi except where the rule provides for alternative.
The labeler must submit product information concerning devices to fda s global unique device identification database gudid unless subject to an exception or alternative.
Erg under contract to fda and are presented in the full report unique device identification udi for medical devices.
Economic analysis of the final rule 2013 ref.
Form and content of the unique device identifier udi draft guidance for industry and food and drug administration staff 08 14 2016 gudid submission.