The unique device identification udi is a system used to mark and identify medical devices within the healthcare supply chain.
Fda unique device identification regulation.
This document will assist industry particularly labelers as defined under 21 cfr 801 3 and fda staff in understanding fda s requirements for direct marking of devices for unique device.
The global unique device identification database gudid is a database administered by the fda that will serve as a reference catalog for every device with a unique device identifier udi.
The us food and drug administration fda released in september 2013 a udi rule which establishes a udi system applying to all medical devices placed on the us market.
830 310 information required for unique device identification.
830 220 termination of fda service as an issuing agency.
Unique device identification system.
The fda established the unique device identification system to adequately identify medical devices sold in the united states from manufacturing through distribution to patient use.
830 320 submission of unique device identification information.
Subpart e global unique device identification database 830 300 devices subject to device identification data submission requirements.
Form and content of the unique device identifier udi draft guidance for industry and food and drug administration staff 08 14 2016 gudid submission.
The fda established the unique device identification system to adequately identify medical devices sold in the united states from manufacturing through distribution to patient use.
A draft version of this.
On 17 december 2013 gs1 has been accredited by the us fda as issuing agency for unique device identifiers udis.
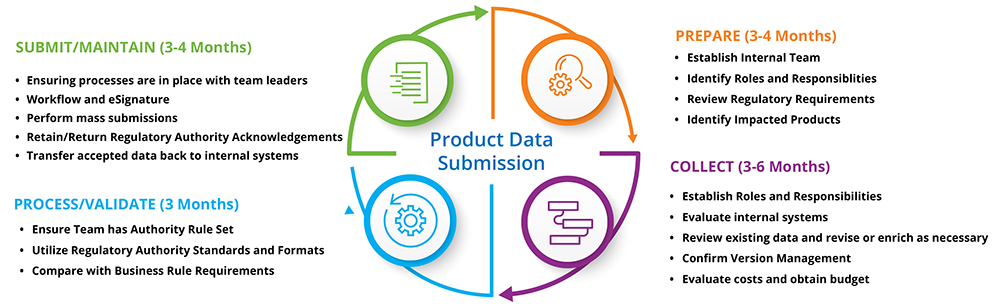
This document is primarily intended for device labelers and provides information necessary for submitting data to the global unique device identification database gudid.